SARS-CoV-2 / Influenza / Respiratory Syncytial Virus PCR
Alias: CoVID-19, Flu A, Flu B, RSV A, RSV B
Discipline: Virology

Test information: A rapid multiplexed real-time RT-PCR test intended for the simultaneous, qualitative detection and differentiation of SARS-CoV-2, influenza A, influenza B, and respiratory syncytial virus (RSV).
Availability: Available locally Monday - Sunday during routine hours: 08:45 - 21:00.
Available out of hours on telephone request.
Turnaround Time: 2 Hours
Related Tests:
- Specimen Type(s)
-
- Nasal Swab;
- Nasopharyngeal Aspirate;
- Nasopharyngeal Swab;
- Other Acceptable Specimen Type(s)
- The performance of nasal/nasopharyngeal wash/aspirates has not been established.
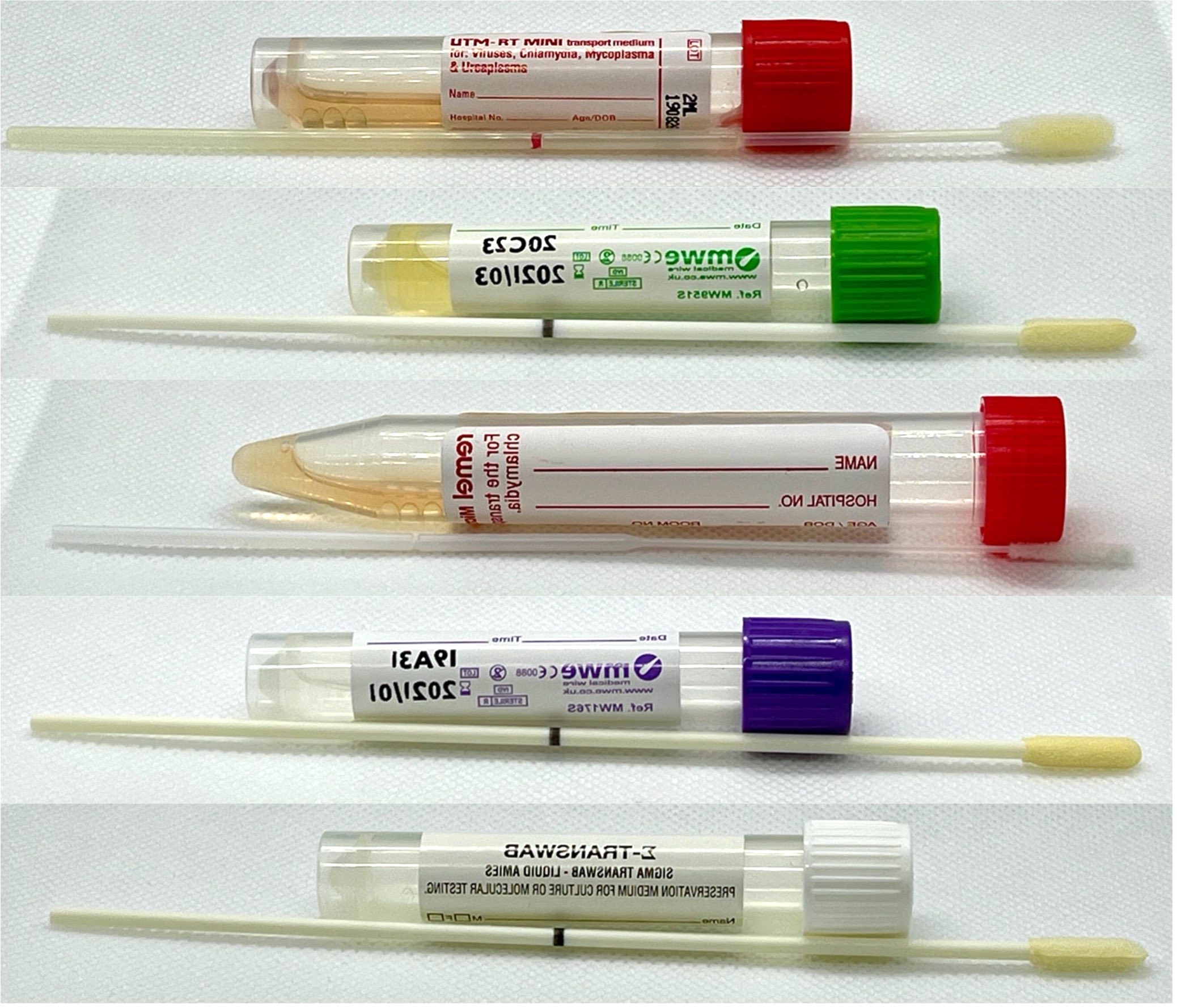
- Specimen Container - Adult
-
- Copan Universal Transport Medium (UTM-RT) Red;
- Remel MicroTest M4RT Red;
- Sigma Transwab Duo MRSA White;
- Sigma Transwab with standard Sigma swab Purple;
- Sigma-Virocult with Sigma-Swab Green;
- Sterile CE-marked Universal Container;
- Specimen Container - Paediatric
-
- N/A;
- Analytes
-
- Influenza A, genes encoding matrix protein, PB2, and PA;
- Influenza B, genes encoding matrix protein and non-structural protein;
- Respiratory Syncytial Virus, genes encoding nucleocapsid of RSV A and RSV B;
- Part of a test profile?
- No
- Volume (min) of sample to be sent to laboratory
- 300uL
- Patient Preparation, Sample Handling and Transport
-
- Proper sample collection, storage, and transport are essential for correct results.
- Specimens can be stored at room temperature (15 °C - 30 °C) for up to 24 hours and refrigerated (2 °C - 8 °C) up to seven days until testing is performed.
- Specimens should be transported at 2 °C - 8 °C.
- Please refer to Trust Policy: Management of Patients with Confirmed/Suspected Influenza Policy (internal only)
- Maximum add on time
- N/A
- Units and Reference Ranges / Interpretation
-
Results: Negative / Positive
Patients that have received a live attenuated nasal vaccine (i.e. Fluenz Tetra / FluMist) prior to specimen collect can result in false positive results.
The performance of this test has not been established for immunocompromised individuals. - UKAS number
- 8869
- UKAS accredited test?
- Yes
- Comments
- N/A
If you have any queries about a test or results interpretation please contact us.
Last updated: 07-06-2023