Chlamydia trachomatis & Neisseria gonorrhoeae NAAT
Alias: CT, Chlamydia, NG, gonorrhoeae, CTNG
Discipline: Virology
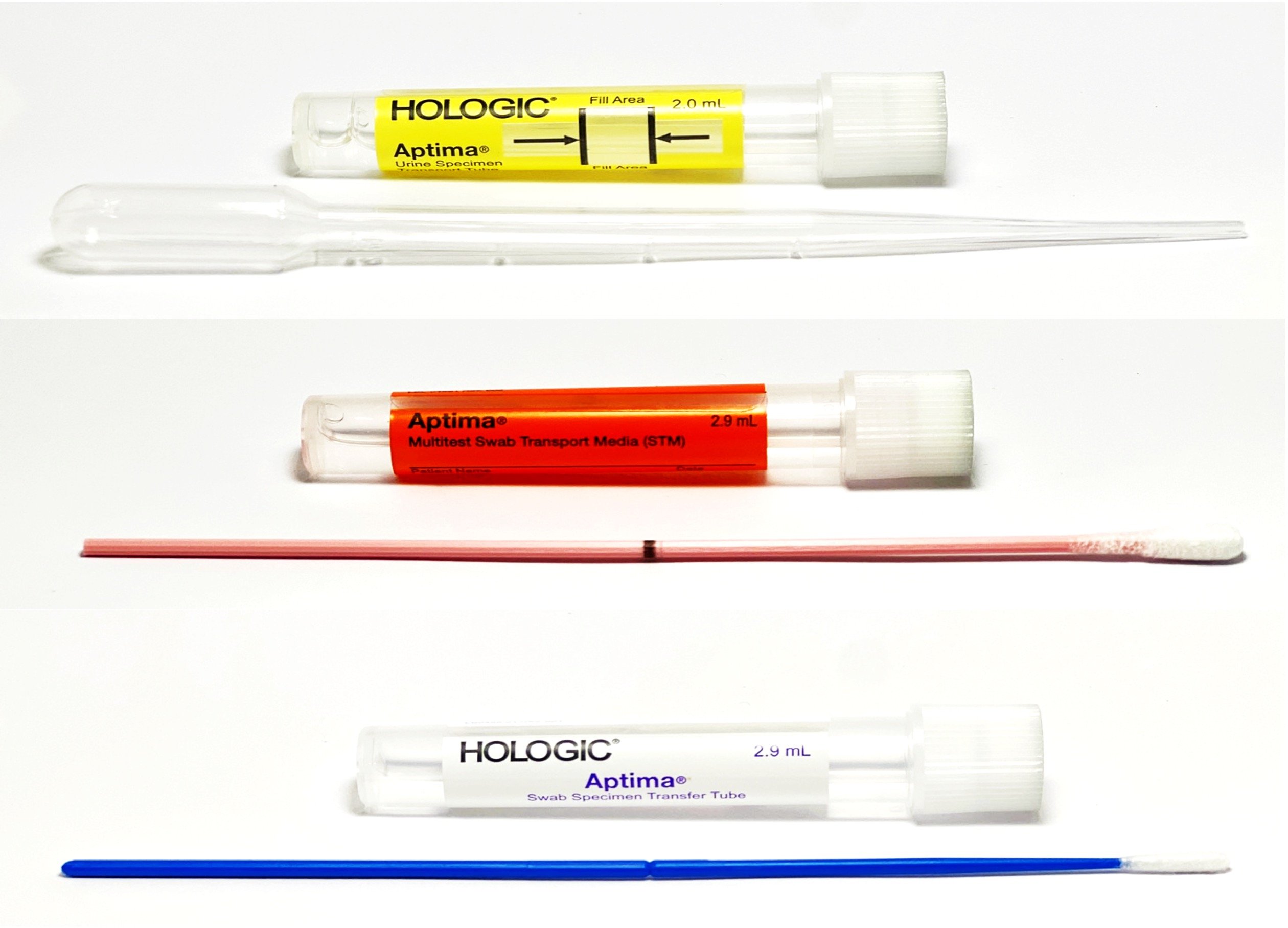
Test information: Qualitative detection and differentiation of Chlamydia trachomatis and/or Neisseria gonorrhoeae to aid in the diagnosis of chlamydial and / or gonococcal urogenital disease.
Availability: Available locally during routine hours: 08:45 - 17:15.
Turnaround Time: 3 Days (NAAT)
- Specimen Type(s)
-
- Endo-cervical Swab;
- Urethral Swab;
- Urine;
- Vaginal Swab;
- Other Acceptable Specimen Type(s)
- N/A
- Specimen Container - Adult
-
- Aptima (Female) Collection Kit / Aptima (Male) Collection Kit;
- Aptima Multitest Swab Specimen Collection Kit;
- Specimen Container - Paediatric
-
- N/A;
- Analytes
-
- Chlamydia trachomatis rRNA;
- N/A;
- Neisseria gonorrhoeae rRNA;
- Part of a test profile?
- No
- Volume (min) of sample to be sent to laboratory
- 2mL
- Patient Preparation, Sample Handling and Transport
-
- Female patients should not cleanse labial area prior to providing urine specimen.
- First catch urine should be collected first in a sterile container free of any preservatives. Care should be taken when transferring urine to the Aptima urine transport tube. The correct volume of urine has been added when fluid level is between black fill lines on urine specimen transport tube label.
- Aptima urine specimens should be transported 2 °C to 30 °C and are stable for up to 30 days prior to testing.
- Swab specimens should be transported 2 °C to 30 °C and are stable for up to 60 days prior to testing.
- Always break the swab shaft at the score line.
- Do not pierce the silver cap.
- Specimens should be transported to the laboratory as soon as possible.
- Maximum add on time
- N/A
- Units and Reference Ranges / Interpretation
- Negative / Equivocal / Positive (Unconfirmed) / Positive (Confirmed)
- UKAS number
- 8869
- UKAS accredited test?
- Yes
- Comments
- N/A
If you have any queries about a test or results interpretation please contact us.
Last updated: 02-06-2023